DOSING INFORMATION

MULTIPLE DOSING OPTIONS WITH FLEXIBILITY
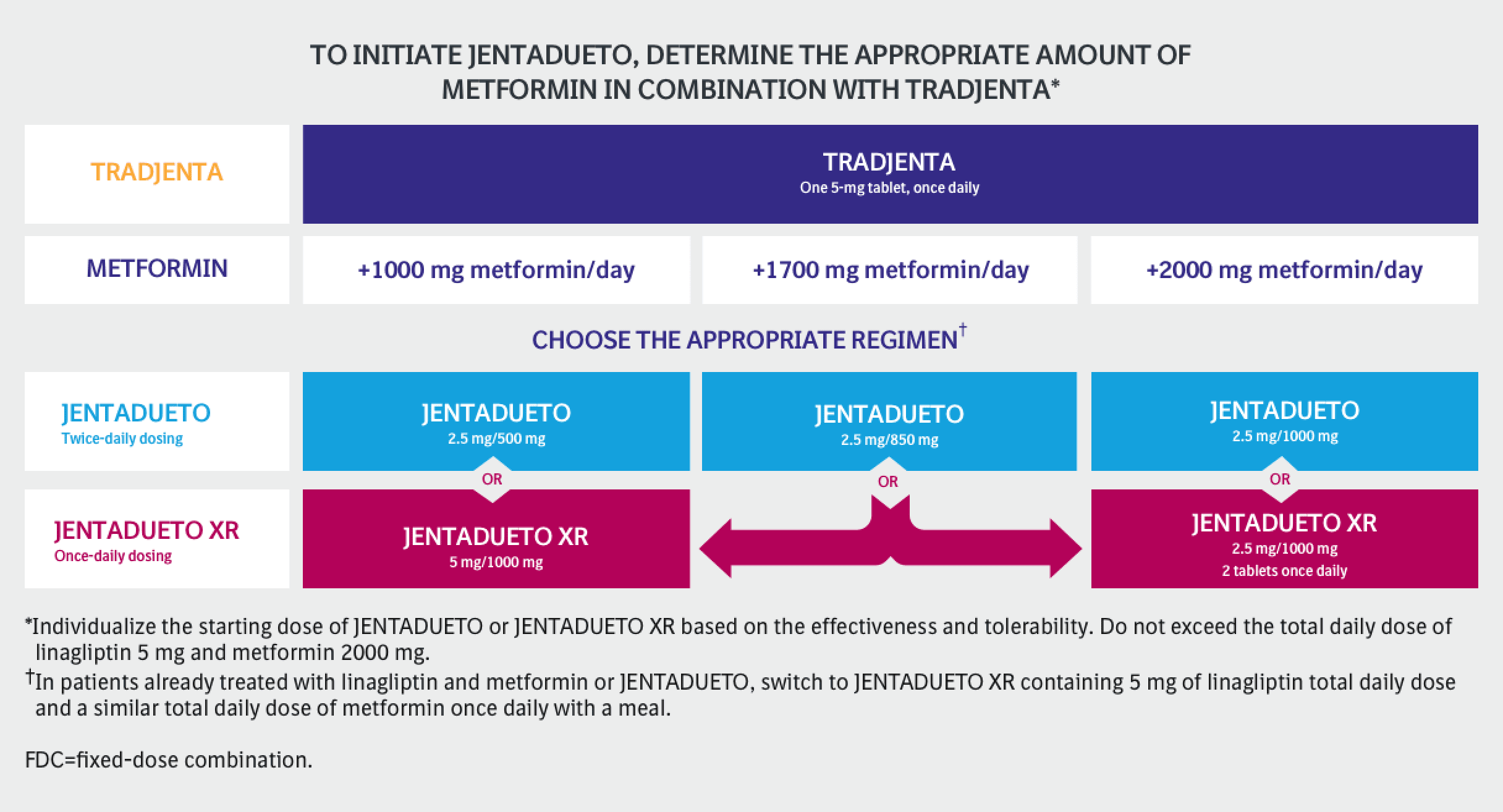
DOSING FOR JENTADUETO
The dosage of JENTADUETO should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended dose of 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily. JENTADUETO should be given twice daily with meals with gradual dose escalation to reduce the gastrointestinal side effects of metformin.
RECOMMENDED STARTING DOSE
-
In patients not currently treated with metformin, initiate JENTADUETO treatment with 2.5 mg linagliptin/500 mg metformin hydrochloride twice daily
-
In patients already treated with metformin, start with 2.5 mg linagliptin and the current dose of metformin taken at each of the two daily meals (e.g., a patient on metformin 1000 mg twice daily would be started on 2.5 mg linagliptin/1000 mg metformin hydrochloride twice daily with meals)
-
Patients already treated with linagliptin and metformin individual components may be switched to JENTADUETO containing the same doses of each component
No studies have been performed specifically examining the safety and efficacy of JENTADUETO in patients previously treated with other oral antihyperglycemic agents and switched to JENTADUETO. Any change in therapy should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
INSULIN SECRETAGOGUES OR INSULINS
Coadministration of JENTADUETO with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the insulin secretagogue or insulin to reduce the risk of hypoglycemia.
MONITORING OF RENAL FUNCTION
-
Before initiation of therapy with JENTADUETO and at least annually thereafter, renal function should be assessed and verified as normal. In patients in whom development of renal impairment is anticipated (e.g., elderly), renal function should be assessed more frequently and JENTADUETO discontinued if evidence of renal impairment is present
-
Radiological studies and surgical procedures: JENTADUETO should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure necessitating restricted intake of food or fluids, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been confirmed to be normal
DOSING FOR JENTADUETO XR
The dosage of JENTADUETO XR should be individualized on the basis of both effectiveness and tolerability, while not exceeding the maximum recommended total daily dose of linagliptin 5 mg and metformin hydrochloride 2000 mg. JENTADUETO XR should be given once daily with a meal.
RECOMMENDED STARTING DOSE
-
In patients currently not treated with metformin, initiate JENTADUETO XR treatment with 5 mg linagliptin/1000 mg metformin hydrochloride extended-release once daily with a meal
-
In patients already treated with metformin, start JENTADUETO XR with 5 mg of linagliptin total daily dose and a similar total daily dose of metformin once daily with a meal
-
In patients already treated with linagliptin and metformin or JENTADUETO, switch to JENTADUETO XR containing 5 mg of linagliptin total daily dose and a similar total daily dose of metformin once daily with a meal
JENTADUETO XR should be swallowed whole. The tablets must not be split, crushed, dissolved, or chewed before swallowing.
No studies have been performed specifically examining the safety and efficacy of JENTADUETO XR in patients previously treated with other oral antihyperglycemic agents and switched to JENTADUETO XR. Any change in therapy should be undertaken with care and appropriate monitoring as changes in glycemic control can occur.
RECOMMENDED DOSING IN RENAL IMPAIRMENT
Assess renal function prior to initiation of JENTADUETO XR and periodically thereafter.
JENTADUETO XR is contraindicated in patients with an estimated glomerular filtration rate (eGFR) below 30 mL/min/1.73 m2.
Initiation of JENTADUETO XR in patients with an eGFR between
30-45 mL/min/1.73 m2 is not recommended.
In patients taking JENTADUETO XR whose eGFR later falls below
45 mL/min/1.73 m2, assess benefit risk of continuing therapy.
Discontinue JENTADUETO XR if the patient’s eGFR later falls below 30 mL/min/1.73 m2.
DISCONTINUATION FOR IODINATED CONTRAST IMAGING PROCEDURES
Discontinue JENTADUETO XR before or at the time of an iodinated contrast imaging procedure in patients: with an eGFR between 30 and 60 mL/min/1.73 m2; with a history of liver disease, alcoholism or heart failure; or who will be administered intra-arterial iodinated contrast. Re-evaluate eGFR 48 hours after the imaging procedure; restart JENTADUETO XR if renal function is stable.
INSULIN SECRETAGOGUES OR INSULINS
Coadministration of JENTADUETO XR with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower doses of the insulin secretagogue or insulin to reduce the risk of hypoglycemia.



